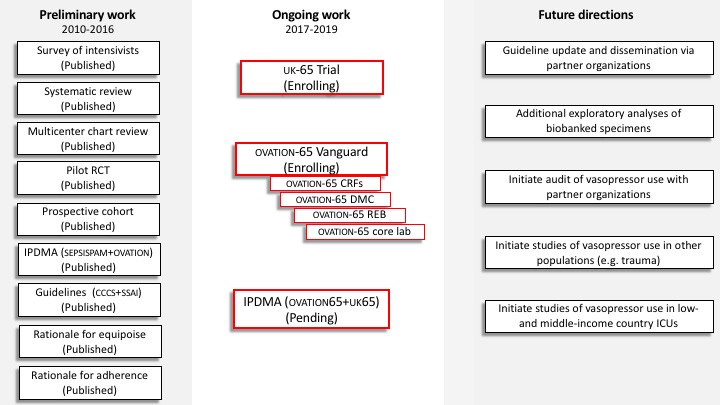
OVATION Program
The goal of the OVATION program of research is to determine a safe blood pressure target for most patients with vasodilatory hypotension requiring vasopressors.
 There is a lack of clinical research to guide the treatment of hypotension in critical illness with vasopressors, drugs that can reverse hypotension. Unfortunately, the safety profile of vasopressors and the extreme vulnerability of critically-ill patients leave treating physicians no margin of error. We have an opportunity with the OVATION program to advance our understanding of, and to translate into practice, the optimal strategy to improve outcomes for these patients.
There is a lack of clinical research to guide the treatment of hypotension in critical illness with vasopressors, drugs that can reverse hypotension. Unfortunately, the safety profile of vasopressors and the extreme vulnerability of critically-ill patients leave treating physicians no margin of error. We have an opportunity with the OVATION program to advance our understanding of, and to translate into practice, the optimal strategy to improve outcomes for these patients.
Research Question: Among critically ill adults aged 65 years and older with vasodilatory hypotension, does minimizing vasopressor use by applying lower MAP targets (60-65 mmHg) vs. usual care reduce the proportion of patients who are either dead or dependent on life-sustaining therapies at day 28?
Preliminary Studies
OVATION Survey
OVATION Retrospective Cohort Study
Vasopressor Stewardship
OVATION Pilot Trial
The OVATION Pilot Trial was completed more than one year ahead of schedule! This study addressses the feasibility of the larger definitive study (see below). The pilot enabled us to develop and test implement measures to optimize protocol adherence for the study. We have established an efficient infrastructure for both Canadian and international participation in the study.
For further information on the pilot refer to our Pilot Trial page.
OVATION Observational Trial
The OVATION Observational trial, was conducted in parallel to the Pilot study, and is also completed. The purpose of the Observational trial was to describe standard practices in the ICU with regards to vasopressor dosing. Across 6 Canadian centers, the trial recruited 60 critically ill, hypotensive adults receiving vasopressors.
OVATION Trial (Definitive Study)
The results of this trial will provide a stronger foundation for establishing the most suitable treatment strategy for critically ill adults aged 65 years and older with vasodilatory hypotension.
This trial will be conducted under the auspices of the Canadian Critical Care Trials Group, in partnership with the Canadian Critical Care Society (CCCS), the Canadian Association of Critical Care Nurses (CACCN) and the Institute for Safer Medication Practices (ISMP) Canada. Other organization support the OVATION Program and will play specific roles moving forward such as the Canadian Society of Hospital Pharmacists (CSHP), the Canadian Association of Emergency Physicians (CAEP), and the Canadian Agency for Drugs and Technologies in Health (CADTH). We are welcoming input from ICU survivors and healthy citizens.
Objectives
The overarching goal of this randomized controlled trial (RCT) of permissive hypotension vs. usual blood pressure targets in hypotensive patients ≥65 years old is to determine whether permissive hypotension can reduce the risk of harm associated with more aggressive vasopressor therapy. The proposed RCT has nine specific objectives which is to evaluate the effect of permissive hypotension on mechanistic outcome: 1) markers of organ injury, primarily in the heart, secondarily in the brain, kidney, liver, intestine, and skeletal muscle; 2) global tissue dysoxia, assessed by serum lactate; 3) organ function, assessed by the multiple organ dysfunction score (MODS); 4) immune response; 5) resource utilization, 6) pre-specified adverse events, 7) the genetic traits as well as the epigenetic marks (methylation and miRNA) of adrenergic receptors and the potential biomarkers of organ injury determined at aim 2; 8) 90-day and 6-month mortality and 6-month cognitive impairment in survivors and 9) the association between baseline level of ascorbic acid and biomarkers of organ injury.
Study Design
Canadian multicentre parallel group RCT embedded in the OVATION-65 international collaboration.
Setting
The Unité de Recherche Clinique et Épidémiologique (URCE) is the Coordinating Centre for this trial in the ICUs across Canada.
Study population
200 subjects, male or female, 65 years old or older, with a diagnosis of vasodilatory hypotension which vasopressors were started ≤ 12 hours and before randomization are expected for ≥ 6 additional hours.
Study Intervention
The intervention is permissive hypotension to carefully minimize dose and duration of vasopressors. For participants allocated to this arm, treating teams will adjust vasopressors to a target MAP range of 60 to 65 mmHg, avoiding vasopressor-induced MAP above this range.
The duration of the trial intervention will be determined by the duration of the hypotensive episode up to a maximum of 28 days. The hypotensive episode will end when vasopressors are discontinued for 24 consecutive hours.
Outcomes
- Mean peak high-sensitivity cardiac troponin (hs cTn)
- Mean peak concentration of biomarkers associated with tissue injury to the:
- brain (GFAP, UCHL1, Myelin Basic Protein and NSE),
- kidney (renal biomarker TBD),
- liver (serum ALT),
- intestine (serum fatty acid binding protein (FABP))
- skeletal muscle (serum CKM)
- cardiac wall stress (serum N-terminal pro-B-type natriuretic [NT- proBNP])
- Global tissue dysoxia will also be assessed through serum lactate.
- Organ function using SOFA score
- Atrial fibrillation (will rely on the ascertainment of clinically detected supraventricular arrhythmia)
- Acute healthcare utilization by measuring durations of:
- mechanical ventilation,
- renal replacement therapy
- vasopressor therapy
- ICU and hospital stay.
- Mortality at 28 and 90 days and the incidence of the prespecified adverse events of stroke, acute kidney injury (KDIGO stage 3), and limb or intestinal ischemia as defined in the OVATION pilot trial.
- Baseline ascorbic levels
- Impact of vasopressor regimen on the immune response and related proteomic profile (IL-1 and TNFa).
- Genetic traits, inherited or shaped by the environment (epigenetic – methylation and miRNA) of the OVATION-65 participants in relation to the catecholamine receptors or the biomarkers determined above.
ENROLLING SITES
| INSTITUTION | # of rand. |
| CIUSSS de l'Estrie-CHUS | 99 |
| Sunnybrook Health Sciences Centre | 11 |
| Centre Hospitalier Universitaire de Montréal | 13 |
| The Ottawa Hospital | 24 |
| CHU de Québec Université-Laval | 6 |
| Toronto Western Hospital | 0 |
| Mount Sinai Hospital | 3 |
2018
Target Blood Pressure for Septic and Vasodilatory Shock
OVATION Pooled Analysis
Permissive Hypotension: Equipoise
2017
OVATION Single Center Retrospective Study
OVATION Systematic Review
OVATION Protocol for a Systematic Review
Expert Versus Patient Perspectives on Vasopressor Therapy for Shock
OVATION Multicentre Prospective Observational Study
OVATION Overview of the Current Methodology and Case Study
Clinical Practice Guideline: Vasopressor Blood Pressure Targets in Critically Ill Adults with Hypotension
Clinical Pratice Guideline: Vasopressors un Early Traumatic Shock
Clinical Practice Guideline: Vasopressor Blood Pressure Targets in Critically Ill Adults with Hypotension and Vasopressor Use in Early Traumatic Shock
Septic Shock Resuscitation in the First Hour
2016
OVATION Multicentre Pilot Randomized Controlled Trial
2015
OVATION Systematic Review
2014
Vasopressor Stewardship
2013
OVATION Retrospective Cohort Study
2011
OVATION Survey
Clinical Trials NCT03431181
www.clinicaltrials.gov
Canadian Agency for Drugs and Technologies in Health
http://www.cadth.ca/
Canadian Association of Critical Care Nurses
http://www.caccn.ca/
Canadian Critical Care Society (CCCS)
http://www.canadiancriticalcare.org/
Canadian Society of Internal Medicine
http://www.csim.ca/
Canadian Society of Hospital Pharmacists
http://www.cshp.ca/
C4 Committee for Critical Care in the Emergency Department
http://www.emicu.org/Canadian_Critical_Care_Practice_Group/Home.html
Institute for Safety Medication Practices
http://www.ismp-canada.org/
United States Critical Illness and Injury Trials Group (USCIITG)
http://www.usciitg.org/
Principal Investigators
 Dr. François Lamontagne
Department of Internal Medicine, Université de Sherbrooke
CIUSSS de l'Estrie-CHUS
Contact Info
3001 12e avenue N. Sherbrooke, QC J1H 5N4
francois.lamontagne@usherbrooke.ca
Dr. François Lamontagne
Department of Internal Medicine, Université de Sherbrooke
CIUSSS de l'Estrie-CHUS
Contact Info
3001 12e avenue N. Sherbrooke, QC J1H 5N4
francois.lamontagne@usherbrooke.ca
 Dr. Neill KJ Adhikari
Department of Critical Care Medicine, Sunnybrook Health Sciences Centre
University of Toronto
Contact Info
2075 Bayview Ave Toronto, ON M4N 3N5
neill.adhikari@sunnybrook.ca
Dr. Neill KJ Adhikari
Department of Critical Care Medicine, Sunnybrook Health Sciences Centre
University of Toronto
Contact Info
2075 Bayview Ave Toronto, ON M4N 3N5
neill.adhikari@sunnybrook.ca
 Dr. M. Elizabeth Wilcox
University Health Network, Toronto Western Hospital
University of Toronto
Contact Info
399 Bathurst Street, 2nd Flr McL, Rm 411-M Toronto, ON M5T 2S8
elizabeth.wilcox@uhn.ca
Dr. M. Elizabeth Wilcox
University Health Network, Toronto Western Hospital
University of Toronto
Contact Info
399 Bathurst Street, 2nd Flr McL, Rm 411-M Toronto, ON M5T 2S8
elizabeth.wilcox@uhn.ca
Research Coordinators
 Marie-Hélène Masse inh
Project Manager, Centre de recherche CIUSSS de l'Estrie - CHUS
Université de Sherbrooke
Contact Info
3001, 12e Avenue N Sherbrooke, QC J1H 5N4
marie-helene.masse.ciussse-chus@ssss.gouv.qc.ca
Marie-Hélène Masse inh
Project Manager, Centre de recherche CIUSSS de l'Estrie - CHUS
Université de Sherbrooke
Contact Info
3001, 12e Avenue N Sherbrooke, QC J1H 5N4
marie-helene.masse.ciussse-chus@ssss.gouv.qc.ca
 Marie-Claude Battista PhD
Operational Manager, Unité de Recherche Clinique et Épidémiologique (URCE)
Centre de recherche CIUSSS de l'Estrie - CHUS, Université de Sherbrooke
Contact Info
3001, 12 Avenue N
Sherbrooke, QC, J1H 5N4
marie-claude.battista@usherbrooke.ca
Marie-Claude Battista PhD
Operational Manager, Unité de Recherche Clinique et Épidémiologique (URCE)
Centre de recherche CIUSSS de l'Estrie - CHUS, Université de Sherbrooke
Contact Info
3001, 12 Avenue N
Sherbrooke, QC, J1H 5N4
marie-claude.battista@usherbrooke.ca
 Marie-Ève Thibault
Research Assistant, Unité de Recherche Clinique et Épidémiologique (URCE)
Centre de recherche CIUSSS de l'Estrie - CHUS
Contact Info
3001, 12 Avenue N Sherbrooke, QC, J1H 5N4
marie-eve.thibault.ciussse-chus@ssss.gouv.qc.ca
Marie-Ève Thibault
Research Assistant, Unité de Recherche Clinique et Épidémiologique (URCE)
Centre de recherche CIUSSS de l'Estrie - CHUS
Contact Info
3001, 12 Avenue N Sherbrooke, QC, J1H 5N4
marie-eve.thibault.ciussse-chus@ssss.gouv.qc.ca
Contact
Do you have any ideas or suggestions about information that you would find helpful to see on this website, please contact one of our:
Principal Investigators
Dr. François Lamontagne
Dr. Neill Adhikari
Dr Elizabeth Wilcox
Project Leaders
Marie-Hélène Masse
Marie-Claude Battista
Thanks to all Participating Centres and collaborators for engaging in this research program!
Thanks to the CCCTG for endorsing this research project.
Clinical Trials NCT03431181