Maureen O. Meade is a critical care consultant at Hamilton Health Science and a professor of Medicine at McMaster University, Canada. A senior clinical investigator of the Canadian Critical Care Trials Group, she has spearheaded 5 randomized controlled trials in lung protective ventilation strategies for ARDS, including the international Lung Open Ventilation Study of higher vs lower PEEP (JAMA 2007) and the international OSCILLATE trial of high frequency oscillation (NEJM 2013). Dr. Meade’s research has advanced clinical practice, with results that have impacted recommendations on UptoDate and recent ARDS management guidelines (AJRCCM 2017).
Dr. Meade is Director of the DONATE Research Program, which she launched together with Dr. D’Aragon in 2015. Their primary objective is to build a national platform for clinical trials in donation science - the intensive care management of deceased organ donors. This platform currently consists of the national cohort study (Canada-DONATE), qualitative research, surveys, feasibility studies and knowledge translation initiatives, to which pilot RCTs and pragmatic, national RCTs will be added in the future. The recipient of local and national research mentorship awards, Dr. Meade’s aim is to foster junior investigators and research trainees in leadership roles on every project of this national collaboration.
DONATE

An Observational Study on Clinical Practices in Deceased Organ Donation
Organ donation rates have been improving in Canada over the past 5 years. We can continue to improve in the area of deceased donation, where Canada rates 20th. Improving/optimizing medical care of deceased donors could improve both the number and function of organs recovered.

Our Goal: Improved Care of Deceased Donors
Our goal is to improve the ICU care of deceased organ donors to help save lives and to improve the health of those who receive organ transplants. We will study, for critically ill adults who die in Canada and then become organ donors, what tests are done to confirm death, what treatments are administered to maintain viability of organs for transplant, and how suitability of the organs for transplant is determined. Moreover, we will assess the extent to which these practices differ across hospitals, and across provinces, and whether these differences help or hinder the rate of organ donation, the rate of organ transplant and the function of transplanted organs in the recipients.
Study Design
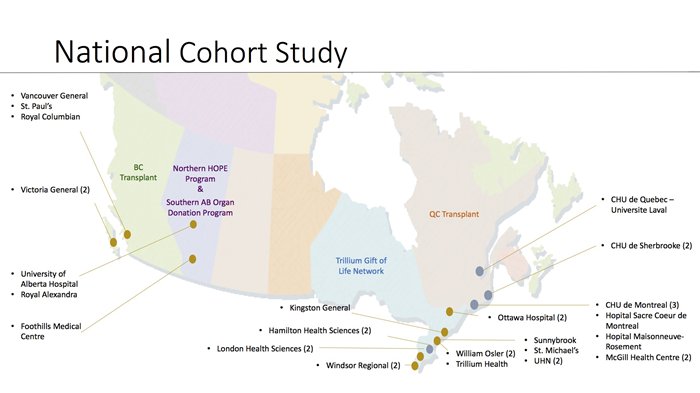
Canada-DONATE is a national, prospective, observational cohort study of organ donation practices. It took place over the course of a 12-month period in each of the 32 highest-volume organ donation hospitals in Canada. 622 consecutive organ donors in intensive care units of active organ donation centres were recruited, using a waiver of research consent, upon consent for organ donation.
Study team leaders
Frédérick D’Aragon, MD, MSc (Epid), MSc (TPM), FRCPC is an Assistant Professor in the Departments of Anesthesiology and Critical Care at the Centre Hospitalier Universitaire de Sherbrooke (CHUS), Quebec. He is a junior investigator and recently obtained a Fonds de Recherche en Santé-Québec Career Award J1. Dr D’Aragon has a unique expertise; in addition to his clinical training in anesthesiology and critical medicine, he holds 2 master degrees: one in Health Research Methodology (McMaster University) and the other in Transplantation Medicine (University of Barcelona).
Dr D’Aragon is co-leading the Canada-DONATE National Cohort Study which aims to describe the management of organ donors across Canada. The results of this study will assess prognostic factors associated with increased organ recovery and will also overcome research barriers and build a national research platform to support clinical trials in organ donation.
Dr. Oczkowski is a critical care physician at Hamilton Health Sciences and an assistant professor in the Department of Medicine at McMaster University. He holds a master’s degree in bioethics from the University of Toronto and is completing his masters of science in clinical epidemiology at McMaster University. His research interests include ethics and end of life care, including organ donation. He is leadingthe Canada-DONATE qualitative study, a mixed-methods project which aims to describe the complex process of organ donation in the intensive care unit. This project will identify key barriers and facilitators to the practice of organ donation and identify ways to improve the process of organ donor care in the ICU.
Farid is a PhD candidate at McMaster university under the supervision of Dr. Maureen O. Meade and Dr. Gordon Guyatt. He is working with the DONATE Canada team to develop a generic graft outcome measurement tool to be used in future trials aimed at identifying the best management strategies for deceased donors. Farid also is part of the heart failure and cardiac transplant team at Toronto General Hospital. In his spare time, Farid loves to spend time exploring the outdoors (challenging and extreme conditions), and participating in endurance sports.
Dr. Samantha Arora is a critical care physician at the Northern Ontario School of Medicine. She is completing her master’s of science in health research methodology at McMaster University, supported in part through a research fellowship from McMaster’s Clinician Investigator Program. Her interests include end of life care and organ donation. Dr. Arora is leading a knowledge translation initiative of DONATE known as Evidence Bulletins, whereby literature relevant to important donation practices are reviewed and systematically appraised or meta-analyzed. Recommendations and clinical pearls are accessibly summarized on rapid-review cards for clinicians to use at the bedside.
The Consent Study
An important hurdle in organ donor management studies relates to research consent. We have completed a systematic and methodological review of organ donation literature to evaluate research consent models. We will be drafting a proposal to share in focus groups with research ethicists, the Canadian Critical Care Trials Group (CCCTG), the Canadian National Transplant Research Program (CNTRP), Organ Procurement Agencies (OPOs), transplant groups and the Canadian Critical Care Society (CCCS). We hope that this will ensure the integrity of our waived consent approach to research consent and prepare us for potentially more challenging situations of research consent in future clinical trials.
DONATE-KT Project
Site-specific practices from each participating hospital will be compared to the current (2006) Canadian guidelines for organ donor management and practices of all other participating hospitals. We will then provide sites with hospital-specific reports on their performance. In addition we will be issuing Evidence Bulletins; reference documents that will advise participating hospitals on current recommendations for medical interventions used in the care of organ donors.
Quality Assurance (QA) Study
We will compare data we collect on Ontario donors to the data collected by the Ontario organ procurement organization (OPO) the Trillium Gift of Life Network (TGLN). OPOs in Canada are provincial organizations responsible for the evaluation and procurement of organs available for transplant. This comparison will help us to validate our data collection, ensuring the data we collect is correct. In addition this process will help us minimize any duplication efforts that occur during data collection.
The Outcomes Methodology Study
Current provincial databases do not directly link clinical data of donors to their recipients. The Canadian National Transplant Research program is working to create a database that will link donor and recipient identifiers such as a health card number but not clinical data. In addition, no outcome measure exists that can be used to summarize graft function across all organ types. We hope to develop a simple measurement scale for this purpose and to be the first to link clinical data of donors to the functional status of the transplanted organ.
The Clinical Qualitative Study
The aim of this study is to describe and understand clinician's attitudes and perceptions about logistical and social barriers to organ donation. We have designed a qualitative research study that will develop theories of the barriers and potential facilitators of organ.
Site Status
| CENTER | # of rand. |
| 1 - Hamilton General (recruitment completed) | 82 |
| 2 - Juravinski (recruitment completed) | 8 |
| 4 - St. Joseph's (recruitment completed) | 8 |
| 5 - London Victoria (recruitment completed) | 24 |
| 6 - London University (recruitment completed) | 21 |
| 7 - CHUS Fleurimont (recruitment completed) | 34 |
| 8 - CHUS Hôtel Dieu (recruitment completed) | 0 |
| 9 - Hôpital du Sacré-Coeur de Montréal (recruitment completed) | 51 |
| 10 - CHUM - Notre Dame (recruitment completed) | 11 |
| 11 - CHUM - St Luc (recruitment completed) | 28 |
| 12 - CHU de Québec-Université Laval (recruitment completed) | 41 |
| 13 - Kingston general hospital (recruitment completed) | 18 |
| 14 - CHUM - Hôtel-Dieu (recruitment completed) | 2 |
| 15 - CUSM - Royal Victoria Hospital (recruitment completed) | 9 |
| 16 - Hôpital Maisonneuve-Rosemont (recruitment completed) | 10 |
| 17 - CUSM - Montreal General Hospital (recruitment completed) | 6 |
| 18 - Windsor-Oulette Campus (recruitment completed) | 16 |
| 19 - Windsor-Metropolitan Campus (recruitment completed) | 2 |
| 20 - Vancouver General Hospital (recruitment completed) | 22 |
| 21 - St. Paul's Hospital (recruitment completed) | 10 |
| 22 - Royal Columbian Hospital (recruitment completed) | 15 |
| 23 - Victoria General (recruitment completed) | 6 |
| 24 - Royal Jubilee (recruitment completed) | 5 |
| 25 - St-Michaels (recruitment completed) | 25 |
| 26 - Sunnybrook Hospital (recruitment completed) | 28 |
| 29 - Ottawa - Civic (recruitment completed) | 26 |
| 30 - Ottawa - General (recruitment completed) | 1 |
| 31 - Trillium Mississauga (recruitment completed) | 11 |
| 32 - Royal Alexandra Hospital (recruitment completed) | 10 |
| 33 - University of Alberta Hospital (recruitment completed) | 30 |
| 34 - Foothills Medical Center (Calgary) (recruitment completed) | 32 |
| 35 - William Osler - Brampton (recruitment completed) | 28 |
| 36 - William Osler - Etobicoke (recruitment completed) | 1 |
| Total | 622 |
Study Design:
A multi-centre, prospective, observational study of 178 participants.
Setting:
Approximately 8 ICUs in Canada.
Sudy Population:
Deceased organ donors.
Study Intervention:
Data collection obtain by obsevation and review of hospital charts. Limited data are obtained throught relevant organ procurement organizations (OPOs).
Primary outcome:
Feasibility, based on:
1. Implementation of a waived consent model
2. Refinement of data collection procédures for adult intensive care units.
3. Development of efficient links to post-transplantation data.
4. Recipient data collection.
Secondary outcomes:
1. Pratice patterns
2. Knowledge translation
3. Quality assurance
4. Generic graft outcome
5. Therapy evaluation
| Title | Download | Publish date |
|---|---|---|
| Newsletter DONATE ENG July 2018 | Download | 2018-07-13 |
| Newsletter DONATE FR July 2018 | Download | 2018-07-13 |
| Newsletter Donate ENG July 2017 | Download | 2017-08-30 |
| Newsletter Donate FR July 2017 | Download | 2017-08-30 |
| Newsletter Donate ENG Nov 2016 | Download | 2016-12-13 |
| Newsletter Donate FR Nov 2016 | Download | 2016-12-13 |
| Newsletter Donate ENG July 2016 | Download | 2016-08-16 |
| Newsletter Donate FR aug 2016 | Download | 2016-08-16 |
| Newsletter Donate ENG mars 2016 | Download | 2016-06-06 |
| Newsletter Donate FR avril 2016 | Download | 2016-06-06 |
You can access the document centre for research coordinators at this link:
http://donate.recherche.usherbrooke.ca/donate-document-center
To obtain a login to the site, please contact Marie-Hélène Masse at mhmasse.chus@ssss.gouv.qc.ca.
JOURNAL PUBLICATIONS
D’Aragon, F. Burns, K., Yaworski, A., Lucas, A., Arseneau, E., Belley-Cote, E., . . . Meade, MO. for the Canadian Critical Care Trials Group and the Canadian National Research Transplant Program. (2019). Research consent models used in prospective studies of neurologically deceased organ donors: a systematic review. J Empir Res Hum Res Ethics. [accepted]
Foroutan, F., Guyatt, G., Friesen, E., Lozano, L. E. C., Sidhu, A., & Meade, M. (2019). Predictors of 1-year mortality in adult lung transplant recipients: a systematic review and meta-analysis. Syst Rev. 8(1), 131. doi:doi:10.1186/s13643-019-1049-x
https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-019-1049-x
Oczkowski, S. J. W., Arnold, E., Centofanti, J., Durepos, P., Sarti, A., Arseneau, E., . . . Meade, M. O. (2019). A mixed-methods study of organ donation in the intensive care unit: 22 actionable practices to improve organ donation. Can J Anaesth. doi:10.1007/s12630-019-01332-9
https://link.springer.com/article/10.1007%2Fs12630-019-01332-9
Oczkowski, S. J. W., Durepos, P., Centofanti, J., Arsenau, E., Dhanani, S., Cook, D. J., & Meade, M. O. (2019). A multidisciplinary survey to assess facilitators and barriers to successful organ donation in the intensive care unit. Prog Transplant, 1526924819835826. doi:10.1177/1526924819835826
https://journals.sagepub.com/doi/full/10.1177/1526924819835826
D'Aragon, F., Cook, D., Dhanani, S., Ribic, C., Burns, K. E. A., Akhtar, A., . . . Program, Canadian Critical Care Trials Group and the Canadian National Transplant Research. (2018). Hamilton-DONATE: a city-wide pilot observational study of the ICU management of deceased organ donors. Can J Anaesth, 65(10), 1110-1119. doi:10.1007/s12630-018-1179-y
https://link.springer.com/article/10.1007%2Fs12630-018-1179-y
Foroutan F, Alba AC, Guyatt G, et al. Predictors of 1-year mortality in heart transplant recipients: a systematic review and meta-analysis. Heart. 2018;104(2):151-160. doi:10.1136/heartjnl-2017-311435
https://heart.bmj.com/content/104/2/151
Oczkowski SJW, Centofanti JE, Durepos P, et al. Organ donation in the ICU: A document analysis of institutional policies, protocols, and order sets. Intensive Crit care Nurs. 2018;45:58-65. doi:10.1016/j.iccn.2017.12.005
https://www.sciencedirect.com/science/article/pii/S0964339717302434
D’Aragon F, Dhanani S, Lamontagne F, et al. Canada-DONATE study protocol: a prospective national observational study of the medical management of deceased organ donors. BMJ Open. 2017;7(9):e018858. doi:10.1136/bmjopen-2017-018858
https://bmjopen.bmj.com/content/7/9/e018858
D’Aragon F, Belley-Cote E, Agarwal A, et al. Effect of corticosteroid administration on neurologically deceased organ donors and transplant recipients: a systematic review and meta-analysis. BMJ Open. 2017;7(6):e014436. doi:10.1136/bmjopen-2016-014436
https://bmjopen.bmj.com/content/7/6/e014436
ABSTRACTS
Foroutan, F., Clark, K., Malik, A., Buchan, TA., Akhtar, A., & Rigobon, A., Stein, M.,Yepes Nuñez, J., Sidhu, A., Guyatt, GH., Meade, M.O. (2019). Predictors of mortality post lung transplantation: systematic review and meta-analysis. The Journal of Heart and Lung Transplantation, 38(4), S333. doi:10.1016/j.healun.2019.01.841
https://www.jhltonline.org/article/S1053-2498(19)30843-5/abstract
Bansal, R., D'Aragon, F., Danha, M., Naylor, K., & Meade M. (2019, March). Canada-DONATE dataLink survey: linking donor and recipient data in research. Poster presented at the 16thAnnual HEI Research Day, McMaster University. Hamilton, Ontario.
D’Aragon, F., Ribic, C., Gangji, A., Treleaven, D., Breau, R., Ball, . . . Meade, M. (2018, November). Extended criteria kidney donors in Canada-DONATE. Poster presented at the Critical Care Canada Forum, 2018. Toronto, Ontario https://cccf.multilearning.com/cccf/2018/eposters/233438/
Arora, S., Aktar, A., Rochwerg, B., D’Agostino, K., Frenette, AJ., D’Aragon, F., . . . Meade MO. (2018, November). Evidence bulletins: bringing evidence-based recommendations on donor management to the bedside. Poster presented at the Critical Care Canada Forum, 2018. Toronto, Ontario.
https://cccf.multilearning.com/cccf/2018/eposters/233402/
Reed, C., D’Aragon, F., Lamontagne, F., & Meade, MO. (2018, October). Mean arterial pressure (MAP) in the neurologically deceased kidney donor: a Canada-DONATE sub-study. Poster presented at the 2018 Canadian Transplant Summit. Ottawa, Ontario
Frenette A-J, Akhtar A, Qi Z, et al. Vasopressin use in donors after cardiac death: a cohort study. [Abstract 201]. Crit Care Med. 2018;46(1):83. doi:10.1097/01.ccm.0000528220.74252.23
https://journals.lww.com/ccmjournal/Citation/2018/01001/201___VASOPRESSIN_USE_IN_DONORS_AFTER_CARDIAC.167.aspx
Frenette A-J, Akhtar A, Williams V, et al. Inotrope use in potential heart donors: a prospective cohort analysis. [Abstract 213]. Crit Care Med. 2018;46(1):89. doi:10.1097/01.ccm.0000528232.17316.b2
https://journals.lww.com/ccmjournal/Citation/2018/01001/213___INOTROPE_USE_IN_POTENTIAL_HEART_DONORS_A.179.aspx
Chassé M, D’Aragon F, Qi Z, et al. Canadian Practices for the Neurologic Determination of Death. [Abstract 460]. Crit Care Med. 2018;46(1):214. doi:10.1097/01.ccm.0000528478.81807.06
https://journals.lww.com/ccmjournal/Citation/2018/01001/460___CANADIAN_PRACTICES_FOR_THE_NEUROLOGIC.426.aspx
Ball I, D’Aragon F, Akhtar A, et al. Donation after Cardiocirculatory Death Practices of Care: A Canadian Multicenter Study. [Abstract 594]. Crit Care Med. 2018;46:282. doi:10.1097/01.ccm.0000528610.77056.14https://journals.lww.com/ccmjournal/Citation/2018/01001/594___DONATION_AFTER_ CARDIOCIRCULATORY_DEATH.558.aspx
DʼAragon F, Frenette AJ, Cook D, et al. DONATE: A 8 centre pilot study. In Abstracts from the 14th Congress of the International Society for Organ Donation and Procurement - ISODP 2017; Sept. 6-9; Geneva, Switzerland; Transplantation. 2017;101:S6. doi:10.1097/01.tp.0000524976.24314.7e
https://journals.lww.com/transplantjournal/Abstract/2017/08002/DONATE___A_8_centre_pilot_study.12.aspx
D’Aragon F. Dhanani S, Cook DJ, et al. Donate-Pilot Study. In Proceedings from the American Thoracic Society 2017 International Conference; May 19-24; Washington, DC; Am J Respir Crit Care Med 2017;195:A2704.
https://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A2704
D’Aragon, F., Akhtar, A., Arseneau, E., Hand, L., Masse, M-H., Meade, M. (2017, October). Regulatory barriers to research in deceased organ donor care. Poster presented at the Critical Care Canada Forum, Toronto, Ontario. https://cccf.multilearning.com/cccf/2017/eposter/198215/
Foroutan, F., Alba, C., Ribic, C., Sidhu, A., Galvin, Z., Akhtar, A., . . . Meade, M. (2017, September). Preliminary results in the development of a generic transplant function rating system. Presented at the Canadian Society of Transplantation, CST-CTRMS Joint Scientific Meeting, Halifax, Nova Scotia.
D’Aragon F, Dhanani S, Cook DJ, et al. E, Hand L, DONATE-Pilot Study. Presented at the American Thoracic Society 2017 International Conference; May 19-24; Washington, DC.
Oczkowski S, Centofanti J, Durepos P,et al. Organ Donation in the intensive care unit: A document analysis of institutional policies, protocols and order sets. Presented at the Canadian Society of Transplantation, CST-CTRMS Joint Scientific Meeting; Sept 26-29, 2017. Halifax, Nova Scotia.
D'Aragon, F., Dhanani, S., Cook, D., Ribic, C., Cupido, C., Lamontagne, F., . . . Maureen, M. (2017, May). DONATE-Pilot study. Poster presented at the American Thoracic Society 2017 International Conference. Washington, DC.
D’Aragon, F., Yaworski, A., Frenette, A-J., Oczkowski, S., Rochwerg, B., Belley-Côté, E., . . . Meade, M. (2016). The DONATE-Consent study. Crit Care Med, 44(12), 216. doi:10.1097/01.ccm.0000509237.42379.0c
https://journals.lww.com/ccmjournal/Fulltext/2016/12001/559___THE_DONATE_CONSENT_STUDY.522.aspx
Meade, M., D’Aragon, F., Dhanani, S., Arseneau, E., Faroutan, F., Ribic, C., . . . Gyatt, G. (2016, November). A generic measure of graft function for deceased donation research. Poster presented at the Canadian Critical Care Forum. Toronto, Ontario.
https://cccf.multilearning.com/cccf/2016/eposter/150951/
D’Aragon, F., Dhanani, S., Arseneau, E., Cook, D., Ribic, C., Lamontagne, F., . . . Meade, M. (2016, November). Canada-DONATE: a national program of research in deceased organ donation. Poster presented at the Canadian Critical Care Forum. Toronto, Ontario.
https://cccf.multilearning.com/cccf/2016/eposter/150952/
D’Aragon, F., Dhanani, S., Arseneau, E., Cook, D., Ribic, C., Lamontagne, F., . . . Meade, M. (2016, October). Canada-DONATE: a national program of research in deceased organ donation. Poster presented at the Canadian Society of Transplantation, CST-CNTRP-SQT Joint Scientific Meeting. Quebec City, Quebec.
Meade, M., D’Aragon, F., Dhanani, S., Arseneau, E., Faroutan, F., Ribic, C., . . . Gyatt, G. (2016, October). A generic measure of graft function for deceased donation research. Poster presented at the Canadian Society of Transplantation, CST-CNTRP-SQT Joint Scientific Meeting. Quebec City, Quebec.
D’Aragon, F., Yaworski, A., Arseneau, E., Oczkowski, S., Rochwerg, B., Belley-Cote, E., . . . Meade, M. (2016, October). The DONATE consent study. Presented at the Scientific Meeting of the Canadian Society of Transplantation, CST-CNTRP-SQT Joint Scientific Meeting. Quebec City, Quebec.
D'Aragon, F., Agarwal, A., Meade, M., Belley-Cote, E., Frenette, A. J., Lamontagne, F., & Dhanani, S. (2014). Effect of steroids administration on brain dead organ donors and recipients: a systematic review. Crit Care Med 42(12), A1375.
http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&AN=00003246-201412001-00029&LSLINK=80&D=ovft
D’Aragon, F., Argawal, A., Belley Cote, E., Frentte, AJ., Lamontagne, F., Dhanani, S., & Meade M. (2014, October). Effect of steroids administration on organ donors after death by neurological criteria and recipients: a meta analysis. Presented at the Canadian Critical Care Canada Forum. Toronto, Ontario.
https://criticalcarecanada.com/abstracts/2014/effect_of_steroids_administration_on_organ_donors_after_death_by_neurological _criteria_and_recipients_a_meta_analysis.php
General Questions
Q: A DCD patient had all of their organs declined and is no longer considered an organ donor. Do I still keep patient enrolled and record data even though they are now a straight withdrawal of life support?
A: YES! We will capture data for all consented organ donors, regardless of whether they go on to donate organs or not.
Q: The patient consented for organ donation today, what study day does this correspond to?
A: This corresponds to the day of consent (C). You can now collect retrospective data from the day prior (C-1) and prospective data for all days that follow (C+1, C+2…) until organ recovery.
Q: Other studies I have been involved with us 8:00 am to 8:00 pm as the study day, is this true for DONATE?
A: Unfortunately, no. Because all of the data for DONATE is recorded in reference to the day of consent (a calendar day) we decided to make all data correspond to the calendar day. We apologize for any inconvenience.
iDataFax Questions
For questions regarding iDataFax, please see solving and minimizing queries document.
Q: I have adjusted the data on a CRF to solve a query but the field is still blue, what do I do?
A: Once you have adjusted the data to solve a query and you save the page as ‘incomplete’, a pop up box should appear to say, you have resolved a query and may not be able to sign off as final. Once you acknowledge this message you should have the green final button appear. IF NOT, please do not respond to the query. Instead contact the methods centre for assistance.
Q: I have entered all fields on a page, there are no queries, and I still cannot sign off as final. What’s up?
A: There may still be some bugs in the system. If this happens please contact the methods centre for assistance and they will address the issue.
Q: I have entered a lab test result that I know is a true value but DataFax is telling me it is out of range. Why?
A: The ranges we have entered into iDataFax are based off of past experience and may not be 100% accurate for organ donors whose values may be very out of range compared to typical ICU patients. In this case please contact the methods centre to have the range adjusted.
CRF Questions
Q: Concerning glycemic blood work (Form 20.1). If the glycemic lab at 00:00 h and 03:00 h are above 8 mmol/L can I assume the “cumulative number of hours glucose>8 mmol/L” is 3.0 hours?
A: As long as there are no other glucose measurements available within this three-hour time span, yes. Count the estimated time above or below glucose thresholds as out of range until there is a normal blood result.
For example, If there was a normal result at 01:30 hours and again at 07:00 hours, the cumulative number of hours >8 mmol/L would be 5.5 hours. That is 1.5 hours from 00:00h to 01:30h and 4 hours from 03:00h to 07:00h for a total of 5.5 hours.
Q: Concerning Daily Data Form 4.2, Q13, 14 & 15. Do we record only episodes above 10 minutes?
A: All episodes of arrhythmia, hypotension and desaturation per day have to be recorded. If 3 episodes of hypotension occur for example, then “3” should be entered on the Daily Data Form and 3 Hypotension Forms (Forms 23.1 – 23.3) should be completed. The 10-minute definition refers to hypoxemia/desaturation. Any arrhythmia requiring intervention should be reported and any hypotension that lasts more than 5 minutes.
Q: In reference to Daily Ventilation Form 5.1 Q10. We do recruitment maneuvers but we do not have a written and approved protocol in our centre. Do we check “Protocol for organ donation” because the maneuver is being done for the purpose of organ donation?
A: If it is not a protocol (written and approved by a committee), this should not be reported as a “protocol for organ donation”. Instead check off “Other” and specify “informal recruitment protocol”.
Q: Concerning vasopressin, it is often given before consent for organ donation. Do we write the date of the beginning of the perfusion even it if before the organ donation consent?
A: Yes. You should write the date the perfusion was initiated, even if it is started before consent for organ donation.
Q: Concerning the Coenrolment Form 34.1, and the question of “Informed Consent Required?”. As DONATE consent is implicit, how do we fill this section. For example, a study about kidney grafts has a deferred consent (specimens are collected from donors at arrival or at time of consent for donation). If the family consents to research during the time of consent for donation with the OPO, the specimens proceed to analysis. As a result, the time of consent for this study is often when we will out the formal written consent. How would you answer for this study.
A: Considering that the formal/signed consent for organ donation confirms enrolment in DONATE, and that the consent for the mentioned study also occurs at the time of formal consent, you should check off “3 = Yes, SAME TIME AS DONATE”.
Questions for Quebec Sites
Q: Where it is written “local organ donation coordinator”, who does this refer to? The local organ donation nurse? Or the Quebec Transplant Coordinator?
A: This refers to the local organ donation nurse (AKA infirmiere-ressource).
Q: When patients are transferred from other hospitals, there is a lot of missing data (labs, vital signs etc.). How do I handle this situation?
A: Try to collect as much missing data as you can. If something is missing, you can mark it as missing in iDataFax. Make sure to answer “yes” to Question 8 on Form 1.1 “Was patient transferred to this hospital strictly for donor management?"
Questions for Ontario Sites
Q: I received a notification from the Methods Centre that there is a consented donor at my site. Is this confirmation that a formal consent for organ donation was obtained?
A: Yes, the automatic notifications from TGLN are sent to the methods centre once the formal consent is entered into their database.
Study collaborators
Thanks to all our collaborators for their precious contributions:
Contact
The coordination of the DONATE Study is a collaborative effort between Dr. Frédérick D'Aragon and his research team at Centre Hospitalier Universitaire de Sherbrooke (CHUS) and Dr Maureen Meade and her research team at Hamilton General Hospital.
Principal Investigators
 Dr. Frédérick D'Aragon
Département d'Anesthésiologie
Centre Hospitalier Universitaire de Sherbrooke
Contact Info
3001 12e avenue N. Sherbrooke, QC J1H 5N4
frederick.daragon@usherbrooke.ca
Dr. Frédérick D'Aragon
Département d'Anesthésiologie
Centre Hospitalier Universitaire de Sherbrooke
Contact Info
3001 12e avenue N. Sherbrooke, QC J1H 5N4
frederick.daragon@usherbrooke.ca
 Dr. Maureen Meade
Faculty of Health Sciences, McMaster University
Hamilton Health Sciences
Contact Info
HSC-2C10 1280 Main Street West Hamilton, Ontario L8N 4K1
meadema@hhsc.ca
Dr. Maureen Meade
Faculty of Health Sciences, McMaster University
Hamilton Health Sciences
Contact Info
HSC-2C10 1280 Main Street West Hamilton, Ontario L8N 4K1
meadema@hhsc.ca
Study administration
 Ruth Breau
Study Project Manager
CLARITY Research Methods Centre
Contact Info
McMaster University 1280 Main Street W Health Sciences Centre (HSC-2C3) Hamilton ON L8S 3K1
breaur@mcmaster.ca
Ruth Breau
Study Project Manager
CLARITY Research Methods Centre
Contact Info
McMaster University 1280 Main Street W Health Sciences Centre (HSC-2C3) Hamilton ON L8S 3K1
breaur@mcmaster.ca
 Marie-Hélène Masse
Central research coordinator for Quebec sites
Contact Info
Centre Hospitalier Universitaire de Sherbrooke 3001 12e avenue N. Sherbrooke, QC J1H 5N4
mhmasse.chus@ssss.gouv.qc.ca
Marie-Hélène Masse
Central research coordinator for Quebec sites
Contact Info
Centre Hospitalier Universitaire de Sherbrooke 3001 12e avenue N. Sherbrooke, QC J1H 5N4
mhmasse.chus@ssss.gouv.qc.ca
 Lori Hand
Research Coordinator
Contact Info
Hamilton General Hospital 237 Barton St E Hamilton ON L8L 2X2
handlori@hhsc.ca
Lori Hand
Research Coordinator
Contact Info
Hamilton General Hospital 237 Barton St E Hamilton ON L8L 2X2
handlori@hhsc.ca
 Shelley Anderson-White
Data Management Assistant
Contact Info
McMaster University 1280 Main St. W Hamilton ON L8S 4K1 McMaster University Health Sciences Centre
sander@mcmaster.ca
Shelley Anderson-White
Data Management Assistant
Contact Info
McMaster University 1280 Main St. W Hamilton ON L8S 4K1 McMaster University Health Sciences Centre
sander@mcmaster.ca
Contact
If you have any ideas or suggestions about information that you would find helpful to see on this website, please contact one of our Project Leaders:
Thanks to all Participating Centers and collaborators for engaging in this research program!
Thanks to the CCCTG for endorsing this research project.
Thanks to the CDTRP for their precious contributions.