Rapid correction of vitamin D deficiency in pediatric critical illness: A phase III multicentre randomized controlled trial (VITdALIZE-KIDS)
SUMMARY OF THE VITdALIZE-KIDS RESEARCH PROGRAM
The overall aim of the VITdALIZE-KIDS Research Program is to understand the impact of VDD in critically ill children, culminating in a clinical trial to determine if restoring vitamin D status in critically ill children with VDD will improve clinical outcomes.
Our Primary Research Objective:
Our primary research objective is to determine if rapid normalization of vitamin D status in critically ill children with VDD improves health-related quality of life (and survival) at 28 days following administration of loading dose vitamin D.
Our Secondary Research Objective:
Our secondary research objective is to evaluate the impact of rapid normalization of vitamin D status on new and progressive multi-organ dysfunction.
BACKGROUND AND RATIONALE
Approximately 10,000 children are admitted to pediatric intensive care units (PICUs) in Canada each year. These children are at risk for multi-organ dysfunction, prolonged recovery, lengthy rehabilitation, and death. A significant number also experience a long-term decline in functional status and/or health related quality of life. As a consequence of both the initial acute illness and chronic morbidity, the family and societal impact of critical illness is substantial. Novel means of decreasing mortality, facilitating rehabilitation, and reducing long-term morbidity would be of great value to children, their families and the health care system.
Given the established role of vitamin D in the health of organs central to critical illness pathophysiology, it has been hypothesized that vitamin D deficiency could represent a modifiable risk factor for worse clinical outcome following critical illness. Multiple observational studies from the adult and pediatric ICU settings have reported both high deficiency rates and significant associations between VDD and organ dysfunction, health resource utilization and mortality.
Although of concern, the high VDD rate in critically ill Canadian children (60-70%) can be viewed as an opportunity to improve clinical outcome. Based on the extensive evidence available, our research group, and others have postulated that optimization of vitamin D status in deficient critically ill children will modulate illness severity, speed recovery, reduce long-term morbidity and save health care dollars.
In the past few years, results from a number of adult ICU clinical trials have provided evidence suggesting that rapid correction of VDD may improve survival and long-term functional outcome or reduce ICU length of stay. Although of value, findings from adult trials cannot be extrapolated to the pediatric critical care setting given differences in dosing, metabolism, co-morbidities, presenting diagnoses, clinical outcome and mortality rates between critically ill adults and children. Consequently, we believe it is essential that the decision about whether to test for, and rapidly correct VDD in PICU be informed by a well-designed, appropriately powered pediatric trial. We aim to address this knowledge gap by conducting a multi-centre Phase III double-blind RCT (the VITdALIZE-KIDS trial).
STUDIES WITHIN THE VITdALIZE-KIDS PROGRAM
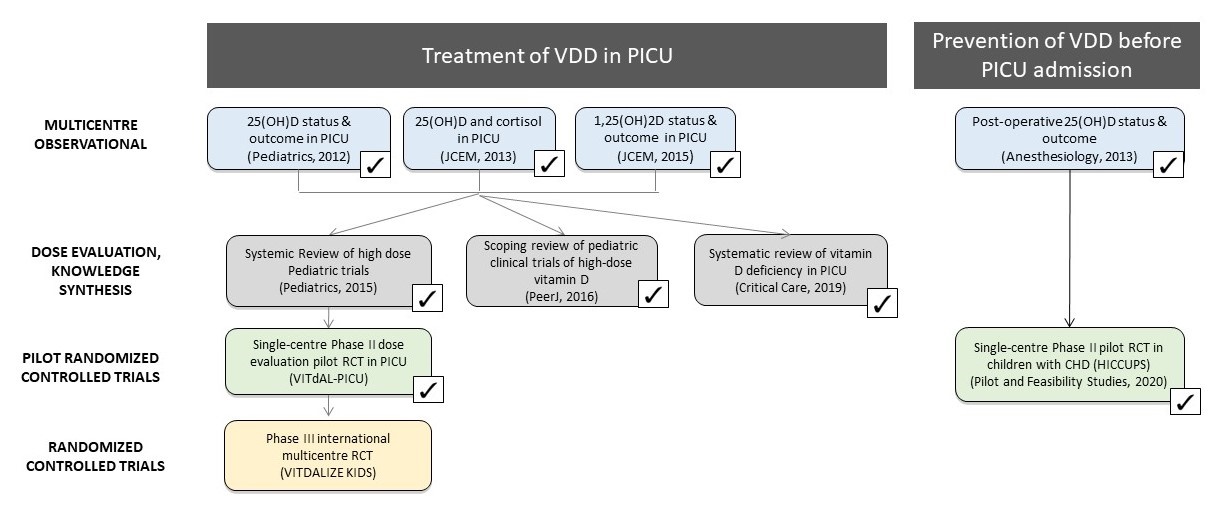
The VITdALIZE-KIDS program is comprised of over 10 years of deliberate and methodical work (Figure 1). We have summarized some of the key studies within the VITdALIZE-KIDS program below.
Figure 1. Overview of the VITdALIZE-KIDS Program (click to enlarge)
OBSERVATIONAL COHORT STUDY - Complete
We conducted a multi-centre observational study to determine the prevalence of VDD, risk factors for its presence, and potential association with clinically relevant outcomes in critically ill children. We found that VDD is both common among critically ill children (~70%) and associated with greater severity of critical illness (hypocalcemia, catecholamine utilization, significant fluid bolus administration, longer PICU length of stay).
View the abstract for this study
SYSTEMATIC REVIEWS - Complete
We performed a systematic review to determine the loading dose most appropriate for a randomized clinical trial evaluating rapid correction of VDD in critically ill children. In this systematic review, we analyzed the 25OHD response in ~100 pediatric high-dose vitamin D clinical trials. Based on meta-analyses, a single load of 10,000 IU/kg (maximum 400,000 IU) was determined as the regimen most likely to rapidly and safely correct VDD.
View abstract of this systematic review
We performed a second systematic review to determine the frequency of VDD in pediatric critical illness and its association with clinical outcomes. We found that approximately 50% of critically ill children have VDD at the time of PICU admission, and that VDD was associated with greater illness severity, multiple organ dysfunction, and mortality.
View abstract of this systematic review
PILOT RANDOMIZED CONTROLLED TRIAL IN CHILDREN WITH CONGENITAL HEART DISEASE (HICCUPS) - Complete
Unlike most patients, children with Congenital Heart Disease (CHD) know ahead of time when they will be admitted to the PICU (i.e. following cardiac surgery). This population presents a unique opportunity to try to prevent VDD before admission to PICU, rather than treating it after admission. We conducted a pilot study to determine whether daily high-dose vitamin D pre-operatively can safely prevent post-operative VDD in children with congenital heart disease. We found that pre-operative daily high-dose supplementation improved vitamin D status pre-operatively and at time of pediatric ICU admission.
View the abstract for the HICCUPS pilot study protocol
View the abstract for the HICCUPS pilot study
DOSE EVALUATION PILOT RANDOMIZATION CONTROLLED TRIAL (VITdAL-PICU)- Complete
We performed a multicenter placebo-controlled phase II dose evaluation pilot randomized clinical trial to determine whether a dosing regimen of 10,000 IU/kg (max 400,000 IU) safely normalized vitamin D status in critically ill children, and to assess the feasibility of proceeding with a phase III trial. We also surveyed participants to help us identify the preferred outcome to use for the phase III trial (health-related quality of life). We found that a single 10,000 IU/kg dose can rapidly and safely normalize blood 25(OH)D concentrations in critically ill children with VDD, but with significant variability in 25(OH)D concentrations. We established that a phase III multicentre trial is feasible, but that using an outcome collected after hospital discharge for the full trial will require strategies to maximize follow-up.
View the VITdAL-PICU pilot study protocol
VITdALIZE-KIDS MAIN RANDOMIZED CONTROLLED TRIAL - Ongoing
VITdALiZE-KIDS is a prospective, randomized, double-blinded, placebo-controlled multi-centre clinical trial examining a single loading dose of vitamin D versus a placebo. Up to 766 children will be enrolled from 11 sites in Canada, and evaluated at baseline, and 28 and 90 days following study enrollment. Our primary objective is to determine if rapid normalization of vitamin D status in critically ill children with VDD improves health-related quality of life (and survival) at 28 days following administration of loading dose vitamin D.
View the list of participating centres and recruitment summary here.
CONTACT US if you are interested in joining this program of research.
Karin Amrein, Philip Britz-McKibbin, Karen Choong, Dean Fergusson, Patricia Fontela, Lindsay Ryerson, Ari Joffe, Pavel Geier, Simon Parsons, Laurie Lee, Anna Gunz, Marisa Tucci, Jennifer Foster, Margaret Lawson, Lauralyn McIntyre, Kusum Menon, Srinivas Murthy, Hope Weiler, Elaine Gilfoyle, Alex Floh, Florence Cayouette
The VITdALIZE-KIDS RCT is part of a larger program on Vitamin D Deficiency (VDD) in pediatric critical illness.
Thanks to all Participating Centres and collaborators for engaging in this research program!
Thanks to the CCCTG for endorsing this research project.