Stress Hydrocortisone in Pediatric Septic Shock (SHIPSS)
Kusum Menon, Michael Agus, Jerry Zimmerman, David Wypij
SUMMARY OF THE SHIPSS PROGRAM
The overall aim of the Stress Hydrocortisone In Pediatric Septic Shock (SHIPSS) Trial is better understand the role of intrinsic and extrinsic corticosteroids in critically ill children culminating in a trial of hydrocortisone in pediatric septic shock.
Primary Research Question for the Main Trial:
Our primary research objective is to determine if hydrocortisone will significantly decrease the number of children with a poor outcome, defined as death or ≥ 25% decrease from baseline in health-related quality of life following septic shock.
Secondary Research Question for the Main Trial:
Our secondary research objective is to determine if hydrocortisone will decrease the number of children who develop new multiple organ dysfunction syndrome following septic shock.
BACKGROUND AND RATIONALE
Management of septic shock is an important clinical problem
Septic shock accounts for ~8% of pediatric intensive care unit (PICU) admissions worldwide (range 6.3% to 23.1%), carries significant morbidity and has a reported mortality rate between 5% and 40% depending on the geographic region in which it occurs. Although fluid, antibiotics and vasoactive agents are the mainstay of therapy, the high morbidity caused by this condition has led some physicians to use corticosteroids as their next line of treatment. Currently, there is no clear evidence for or against the use of corticosteroids for pediatric septic shock. While there is scientific rationale and limited data supporting their use in this setting, there is also data from other populations suggesting potential harm.
Potential adverse effects of corticosteroids in pediatric septic shock
Previous pediatric trials have been too small to detect a difference in the rate of adverse events. One trial reported a statistically significant increase in bleeding with corticosteroids. More recently several larger, mostly retrospective, studies have suggested an increased risk of mortality, secondary infections, PICU length of stay and suppression of adaptive immunity from corticosteroid administration, especially in certain subgroups of patients. Given the lack of clear safety information in the PICU population, it is impossible for clinicians to make evidence-based decisions regarding the risk or benefit of administering corticosteroids to children with septic shock. As a result, some clinicians choose to administer corticosteroids while others do not. The effectiveness and safety of both of these approaches remain to be demonstrated.
Evidence is lacking for or against the use of corticosteroids in pediatric septic shock
Numerous surveys and editorials have emphasized the need for a large, well-designed randomized controlled trial to definitively determine the effect of corticosteroids in pediatric septic shock. Importantly, the results of the SHIPSS Program will be useful regardless of the findings; if a beneficial effect of corticosteroids is identified, this will guide practice; if no benefit is demonstrated, this will prevent needless or harmful use of corticosteroids. In addition, our exploratory outcomes will generate valuable information on potential outcome measures for future pediatric septic shock trials.
STUDIES WITHIN THE SHIPSS PROGRAM
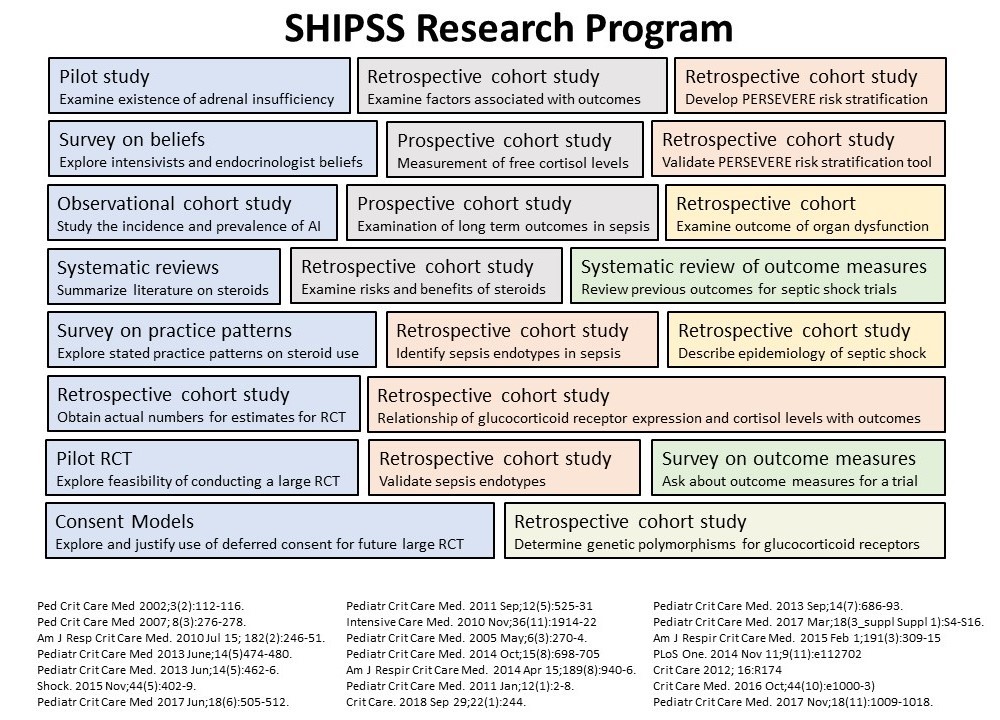
The SHIPSS program is comprised of over 20 years of deliberate and methodical work by several individual members and combinations of members of our team (Figure 1). We have summarized some of the key studies within the SHIPSS program below.
Figure 1. Overview of the SHIPSS Program (click to enlarge)
PILOT STUDY - Complete
We conducted a pilot observational study in 13 critically ill children to examine the existence of adrenal insufficiency, and found that some critically ill patients have adrenal dysfunction. This highlighted the need for future studies to better understand adrenal dysfunction in critically ill children, and its consequences.
View the abstract for this study
SURVEY ON BELIEFS - Complete
We conducted a survey of Canadian endocrinologists and PICU physicians to determine their beliefs and practices regarding adrenal dysfunction in pediatric critical illness. We found that there is no consensus among PICU physicians or endocrinologists as to how often adrenal insufficiency occurs in pediatric critical illness or how to diagnose this condition. Our findings highlighted the need for further studies to determine the definition, frequency, and appropriate treatment of adrenal insufficiency in critically ill pediatric patients.
View the abstract for this study
OBSERVATIONAL COHORT STUDY - Complete
We conducted an observational study in 7 PICUs in Canada. We found that adrenal insufficiency occurs in many disease conditions in critically ill children and is associated with an increased use of catecholamines and fluid boluses, and with high baseline cortisol levels. We concluded that further research was needed to determine which of these critically ill children truly have low cortisol levels before any treatment recommendations can be made.
View the abstract for this study
SYSTEMATIC REVIEW - Complete
We conducted a systematic review to identify all of the existing pediatric RCTs evaluating the use of corticosteroids in pediatric septic shock. We identified eight pediatric RCTs. No individual study included more than 100 patients, and all 8 studies were conducted in the developing world. Our systematic review showed that there is limited evidence to help clinicians decide whether or not to use corticosteroids to treat septic shock, and supported the need for a large pragmatic randomized controlled trial on the use of steroids in pediatric shock to inform future guidelines.
View the abstract of the systematic review
SURVEY ON PRACTICES - Complete
We conducted a survey of PICU physicians from 15 Canadian hospitals. Our survey showed that physicians feel that the role of steroids in shock still requires clarification and that they would be willing to randomize patients into a trial.
View the abstract for this survey
RETROSPECTIVE COHORT STUDY - Complete
We conducted a retrospective chart review at four PICUs in Canada to describe practice patterns and outcomes associated with corticosteroid use in children with shock. We found that corticosteroids administration was associated with longer time on vasopressors and increased incidence of positive cultures even after accounting for illness severity. We also found that corticosteroids administration was not associated with a clear benefit but was associated with increased time on vasopressors and increased incidence of new positive cultures. These findings strongly supported the need for a well-designed, randomized controlled trial on the effect of corticosteroids in pediatric shock to better delineate the risks and benefits of corticosteroid use in this population.
View the abstract for this study
PILOT RANDOMIZED CONTROLLED TRIAL (STRIPES) - Complete
We conducted a pilot feasibility trial (Steroid Use in Pediatric Fluid and Vasoactive Infusion Dependent Shock (STRIPES)) to identify potential barriers to conducting the SHIPSS Main RCT. We showed that we could successfully enroll patients within a narrow 8-hour window, a high rate of protocol adherence and a modest rate of post-randomization open label steroid use. Based on the success of the STRIPES pilot study, we have now moved onto the next stage of this program: the SHIPSS Main RCT.
EVALUATION OF CONSENT MODELS - Complete
During the STRIPES Pilot Trial, we evaluated two different approaches to consent: prior informed consent and deferred consent. STRIPES demonstrated that a deferred consent model was acceptable to the Research Ethics Board (REB) at participating sites, families, health care providers, and research staff. Deferred consent enhanced trial feasibility by allowing earlier randomization and a higher consent rate compared to prior informed consent.
View the abstract comparing the use of prior informed vs deferred consent in STRIPES
SHIPSS MAIN RANDOMIZED CONTROLLED TRIAL - Ongoing
SHIPSS (Stress Hydrocortisone In Pediatric Septic Shock) is a prospective, randomized, double-blinded, placebo-controlled international clinical trial examining the potential benefits and risks of hydrocortisone in critically ill children with septic shock. Up to 1,032 children will be enrolled from sites in Canada, United States, South America, and Asia, and evaluated at baseline, and 28 and 90 days following study enrollment. Our primary hypothesis is that hydrocortisone, compared to placebo, will decrease the proportion of subjects with poor outcomes at Day 28, defined as death or a ≥25% decrease in health-related quality of life.
View the SHIPSS main RCT trial registration
View the SHIPSS main RCT website
SHIPSS Main RCT Core Leadership Committee (CLC): View a list of the current members of the CLC here.
Thanks to all Participating Centres and collaborators for engaging in this research program!
Thanks to the CCCTG for endorsing this research project.
Katie O’Hearn (Canada), Kerry Coughlin-Wells (US)
View the list of participating centres and recruitment summary here.
CONTACT Kusum Menon or Katie O’Hearn if you are interested in joining this program of research.
Hector Wong*, Dayre McNally, Karen Choong, Tim Ramsay, Anand Acharya, Jan Hau Lee, Daniela Carla de Souza, Claudio Flauzino
*We acknowledge Hector’s invaluable contributions to the planning, implementation and conduct of this trial. The SHIPSS main RCT is part of the larger SHIPSS Research Program.